CTC Directors will provide first time users with initial study consultation, an overview of available services, and will facilitate connections to Unit/Subunit directors and scientific liaisons. The CTC offers investigators a wide range of services for research in IDD.
| UNIT | SERVICE | COST / UNIT |
| HGPCU | Consultation (Study Design, Protocols, Analysis)–Free 1st Consult | Free Initial 2-Hour Consultation |
| HGPCU | Calibration, Reliability Autism Assessment | 250 / Hour |
| HGPCU | Training in Standardized Behavioral Assessment | 250 / Hour |
| HGPCU | Specialized Behavioral Phenotyping: Neuropsychology Testing | 250 / Hour |
| HGPCU | Specialized Behavioral Phenotyping: Behavioral Assessment | 150 / Hour |
| HGPCU | Rapid, Virtual Neurobehavioral Assessment | 250 / Individual |
| HGPCU | ERP/EEG | 250 / Hour |
| HGPCU | fNIRS | 250 / Hour |
| HGPCU | Research Support Level I | 35 / Hour |
| HGPCU | Research Support Level II | 65 / Hour |
| HGPCU | QC / variant calling, research whole-exome sequencing | 150 / Individual |
| HGPCU | QC / variant calling, research whole-genome sequencing | 300 / Individual |
| CTU | Clinical Biomaterials Acquisition (CBA) Patient Recruitment | Custom |
| CTU | CBA: Informed Consent | 250 / Individual |
| CTU | CBA: Biomaterials Kits (blood, urine, saliva, stool, tissue) | Custom |
| CTU | CBA: Biomaterials Acquisition | 150 / Hour |
| CTU | CBA Sample Preparation | Custom |
| CTU | EHR Data Abstraction for Natural History Study | 50 / Hour |
| CTU | Clinical Trial Registration / IRB Submission | Custom |
| CTU | UMD Dataset/Consultation | Free Initial Consultation |
| HGPCU | Rental of -80 Freezer Storage | 500 / Per Year per drawer |
| Ethics Consultation Service | Free Initial 2-hour Consultation |
For more information about the services of the Clinical Translational Core, please contact:
HGPCU: Anna Abbacchi: abbaccha@wustl.edu; 314-971-0194
CTU: Angela Keith: keitha@wustl.edu; 314-273-2622
Human Genomic and Phenotypic Characterization Unit
The HGPCU is specifically designed to:
- support investigators seeking consultation and data interpretation with respect to genomic investigation in IDD research;
- oversee the incorporation of genomic data into databases (of investigators and national networks) that link genotypic, phenotypic, neuroimaging, environmental, and clinical data in IDD gene discovery;
- support gene-based clinical research studies;
- acquire and analyze patient biomaterials to establish targets for personalized therapeutic intervention in IDD;
- facilitate consultation and evaluation for deep phenotypic characterization of development, behavior, and psychopathology in IDD patients;
- provide requisite technical expertise, infrastructure, and equipment to facilitate multimodality phenotyping in IDD; and
- assist in the elucidation of the developmental and behavioral consequences of environmental deprivation in IDD.
- The CTC maintains the IDDRC WUSTL Gene Database which reflects current genes that are being studied by IDDRC investigators and connects investigators interested in collaborative studies related to these genes. To participate in this gene database, please complete the following survey.
HGPCU Services
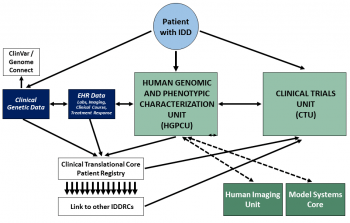
Human genomic characterization coordinated by this unit includes chromosomal microarray, whole exome, and whole genome sequencing; the data is utilized by individual investigators at WUSTL and made available to their collaborators around the world. Positive results are reported to ClinVar/ClinGen, the NIH-led effort to curate genes by accumulated acquisition of individual genotypes and phenotypes for the purpose of specifying pathogenicity and the role of genetic variants in disease processes. The HGPCU also relies upon in-depth phenotypic characterization (see below), as well as clinical data acquired from electronic health records.

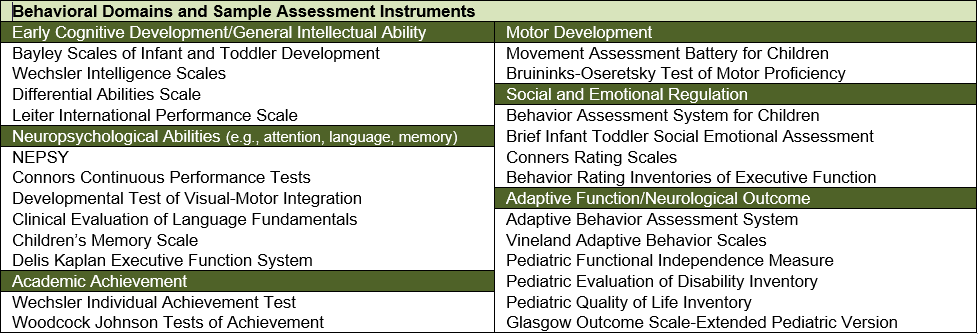
Comprehensive phenotypic characterization of human subjects with or at risk for IDD is the second major focus of the HGPCU, recognizing the need for state-of-the-art phenotypic assessment in understanding genetic and environmental influences on brain and behavioral development. Some of the most compelling technologies for ascertaining developmental liability are often difficult to establish in individual laboratories and can be provided centrally as a highly-valued service by personnel who maintain expertise in the respective methods. The assessment modalities most sought after by scientists in our environment include highly technical observational assessments, as are required in infant research or in the appraisal of autistic syndromes; diagnostic confirmation in the context of comorbidities (e.g., Autism and Intellectual Disability, or Intellectual Disability and ADHD); electrophysiology (EEG), eye tracking, near infrared spectroscopy (NIRS), and characterization of environmental and social factors that impact fetal, infant, and child development. Expert phenotypic characterization occurs in dedicated space adjacent to the Child and Family Development Center of the Division of Child Psychiatry at WUSTL, which features multiple observation rooms with one-way mirrors, state-of-the-art video recording equipment, and dedicated environments for each of the technologies listed above.
The HGPCU supports investigators in their IDD research not only through the acquisition of data using these methods, but also through the provision of consultation on study design and test selection, data processing, cleaning, storage, and data interpretation services, with exceptional expertise related to quantitative behavioral phenotyping and analysis.
In tandem with these efforts and in close coordination with the Human Imaging Unit of the Developmental Neuroimaging Core (DNC), the HGPCU is adapting standard MRI scanning protocols employed at St. Louis Children’s Hospital to leverage advances in scanner technology and sequence development and perform research-quality neuroimaging studies during every clinical scan performed in targeted IDD clinical populations (e.g., neurofibromatosis, Fragile X). This approach both optimizes data quality for clinical teams and provides a rich repository of multimodality data delineating brain structure and function that can be incorporated into investigations of IDD patients. Similar efforts have been successfully implemented in routine clinical practice for multiple populations at St. Louis Children’s Hospital, including children undergoing neurosurgical intervention for epilepsy and infants with hypoxic-ischemic encephalopathy, providing a successful template for expansion into IDD cohorts. Critically, when undertaken in coordination with clinical teams, these procedures can be implemented without altering system workflow, scan duration, and cost.
The HGPCU assists investigators in the complex task of characterizing general psychopathological comorbidities of IDD. Psychopathological co-morbidity is common, often unrecognized, has profound implications for adaptive functioning, and may be one of the most treatable aspects of IDD, if not causal in its own right. The HGPCU offers deep expertise in the analysis of relationships between IDD syndromes and medical and behavioral comorbidity according to evaluation of competing models of overlap.
A linked area of focus includes characterization of clinical and sub-clinical autistic syndromes using state-of-the-art instruments (e.g., Autism Diagnostic Observation Schedule, Autism Diagnostic Interview-Revised, Social Responsiveness Scale) administered and overseen by IDDRC@WUSTL staff psychometrician Anna Abbacchi, who has established proficiency in accordance with currently-accepted standards and certifications for autism research.
In addition, through Dr. Constantino, reference databases are available to investigators that allow targeted comparisons of new data with existing data from large, well-characterized, genetically-informative reference samples, including:
- cohort of male sibling pairs concordant and discordant for autism;
- cohort of siblings of children affected by autism;
- two NIH Autism Centers of Excellence, one in gene discovery, one in infant brain imaging; and
- the Missouri Family Research Center, an epidemiologic sampling platform maintained jointly by the State of Missouri and the WUSTL Department of Psychiatry—Dr. Constantino is a member of the executive committee of the Center.
Social adversity is prevalent in IDD, with environmental deprivation interacting with developmental and genetic liabilities to exert critical influences on child outcomes. The HGPCU interacts with two key allied WUSTL facilities for comprehensive characterization of the prevalence and severity of social determinants of outcome:
- the Women and Infants Health Specimen Consortium (WIHSC) within the Washington University Department of Obstetrics and Gynecology, which has an active tissue procurement program for accrual of key biomaterials reflective of the preconception and prenatal intrauterine environment (e.g., maternal blood, placenta, and cord blood acquired simultaneously); and
- the George Warren Brown School of Social Work, which is internationally-renowned for its access to and capacity for analysis of state administrative databases related to indices of child well-being (e.g., official-report child maltreatment, foster care placement, sociodemographic risk). The Brown School also houses the NICHD-funded Center for Innovation in Child Maltreatment―site of groundbreaking research on childhood maltreatment rates in IDD.
In addition to characterization of causal mechanisms mediated by social risk and the intrauterine environment, translational opportunities for prevention of prematurity and mitigation of social risk arise from deeper understanding of causal mechanisms.
Clinical Trials Unit
The Clinical Trials Unit (CTU) within the CTC reflects the next phase of promotion of IDD discovery in which specific objectives are to:
- to ensure clinical trials “readiness” within the IDDRC@WUSTL [e.g. to enable personalized intervention and clinical trials for IDD including the development of natural history studies];
- enhance and develop novel methods for patient recruitment; and
- support the training and capacity-building for physicians, researchers, and technical staff to perform high-quality intervention research within the IDDRC@WUSTL.
CTU Services
For investigators planning studies on patients with gene-based IDD syndromes, including biomarker studies, natural history studies, registries, and clinical trials, the CTU provides consultative services encompassing IRB and regulatory support and guidance, budget development, recruitment support services, and referral to existing core resources available through the CTSA, including the
Since many existing CTSA resources have little experience with the challenges of IDD research, including recruitment of subjects with rare diseases across multiple institutions, delivery of viral vectors for gene therapy (often intrathecally in sedated pediatric patients with multiple medical needs), and the ethical considerations for studies of vulnerable populations. The CTU fills these critical gaps to facilitate leading-edge IDD research.
In recognition of the insufficient supply of researchers, clinicians, and technical staff with training in leading or conducting human clinical trials in IDD, the CTU works closely with the CTSA to enhance workforce development. CTU leadership coordinates monthly meetings for investigators engaged or wishing to engage in IDD prevention or intervention trials that encompass discussion of unique aspects of research protocols, including recruitment and regulatory requirements for gene-based therapies including gene or enzyme replacement. A similar monthly forum is held for study coordinators engaged in IDD research across Departments, including but not limited to Neurology, Psychiatry, and Pediatrics, to share best practices and regulatory knowledge, supplementing training opportunities available through the CTSA Center for Clinical Studies and Accelerating Clinical Coordinator Excellence Program (ACCE). The IDDRC@WUSTL seminar series features researchers engaged in prevention or treatment trials addressing solutions to the challenges of studying this population.
Early engagement of families and community collaborators is key for understanding the impact of IDD on families and defining optimal goals for intervention trials. Our faculty have significant relationships with family organizations (e.g., RettSyndrome.org; Bow Foundation for study of GNAO1 disorders; Tuberous Sclerosis Alliance; M-CM Network), and we will continue to encourage and train our faculty to align with these key collaborators to support recruitment of patients for natural history studies, registries, and ultimately, clinical trials. The Center for Families at St. Louis Children’s Hospital has also been engaged to assist our faculty with the organizational and logistical support for sponsoring regional and national meetings of these organizations in St. Louis, which also often serve to facilitate research studies (natural history studies, biospecimen acquisition). The CTU supports and links faculty with resources needed to pursue these types of research activities as they are key precursors for successful clinical trials.
The opportunity for patients with rare genetic forms of IDD to participate in disease-specific research initiatives, including model systems studies, clinical trials, and natural history studies (e.g., of response to treatment), depends upon the ability of scientists and families to find one another. In addition, an institutional database of patients with specific monogenic syndromes is maintained by the CTU in collaboration with the Undiagnosed Mendelian Disorders clinic at St. Louis Children’s Hospital. The database is organized by brain gene, is implemented in the Washington University Research Electronic Data Capture System (REDCAP), and incorporates linkage to genomic sequencing data, electronic health record data, and standardized demographic, environmental, imaging, developmental, and behavioral data.
IDDRC Ethics Consulation Services
An Ethics Consultation Service, led by Tristan McIntosh, PhD, is available to IDDRC faculty. Faculty may request a free two-hour ethics consultation. The IDDRC values research that intentionally and proactively takes into account ethical, legal, and social issues at all stages of research. Additionally, one of the primary objectives stated NIH’s 2021-2025 Strategic Plan is, “exemplifying and promoting the highest level of scientific integrity, public accountability, and social responsibility in the conduct of science” (National Institutes of Health, 2021). The IDDRC Ethics Consultation Service is intended to strengthen grant applications and help investigators be responsive to ethics priorities and requirements of the NIH and other funding agencies.
Ethics consultations can help IDDRC faculty identify and think through ethical considerations in their research. This could include identifying and weighing risks and benefits of certain treatments and therapies, how to best communicate test results to patients and caregivers, and anticipating how certain scientific advances will affect certain populations. Ethics consultations can also be leveraged to make grant applications more competitive through integrating ethics aims into research proposals, establishing a Neuroethics Core for large-scale projects, or applying for ethics supplements.
If you are interested in receiving an ethics consultation, please email Dr. Tristan McIntosh directly at t.mcintosh@wustl.edu. In your email please include a brief description of your ethics consultation needs, including relevant information about the project(s), the population(s) you work with, and any time deadline constraints. Dr. McIntosh will then work with you to schedule a zoom call.
References:
National Institutes of Health (2021), 2021-2025 Strategic Plan.